Overview
 Lyme disease (LD), also called Borreliosis or Lyme borreliosis, is a zoonosis, i.e., a bacterial infection transmitted by ticks. It was first recognized in 1975 in Old Lyme, Connecticut, USA, however, reports of it can be found in medical literature in Europe as early as 1883. Statistics confirm that LD has become the most common arthropod-borne (vector-borne) illness in the U.S. (~30,000 reported cases each year) and Europe (~65-80,000 cases reported each year). Its occurrence has been documented on all continents except Antarctica.
Lyme disease (LD), also called Borreliosis or Lyme borreliosis, is a zoonosis, i.e., a bacterial infection transmitted by ticks. It was first recognized in 1975 in Old Lyme, Connecticut, USA, however, reports of it can be found in medical literature in Europe as early as 1883. Statistics confirm that LD has become the most common arthropod-borne (vector-borne) illness in the U.S. (~30,000 reported cases each year) and Europe (~65-80,000 cases reported each year). Its occurrence has been documented on all continents except Antarctica.
Causes: LD is caused by the bacterium of genus Borrelia that is harbored in ticks. In 1981 Willy Burgdorfer established that a particular species Borrelia burgdorferi sensu lato, a type of bacterium called a spirochete (pronounced spy-ro-keet) is responsible for LD. Borrelia can be found frequently in small and large mammals, birds and reptiles on which many ticks feed and mate making them prone to become infected with the bacterial spirochete. An infected tick may then transmit these bacteria to another host such as humans and/or animals by biting them. Some studies have shown that in order to transmit the spirochete an infected tick (also known as the black-legged tick) needs to be attached to the host for 36-48 hours, however, other studies show that attachment of 24-48 hours can be already sufficient. The ticks that spread LD can sometimes co-transmit other tick-borne pathogens such as: Ehrlichia spp., Babesia spp., Bartonella spp., and others.
Diagnosis: LD is diagnosed based on the symptoms described by a patient, such as flu-like symptoms (including fever, headache, and fatigue), physical signs (e.g., rash and swollen areas on the body), and the results of laboratory testing. Because the symptoms of LD can be diverse and mimic other diseases, and laboratory tests are still not 100% reliable, the diagnosis and treatment quite often represents a challenge for clinicians. Untreated LD allows the dissemination of the bacterium through the bloodstream to various body tissues and can lead to developing arthritis, meningitis, inflammation of the brain and heart and others.
Treatment: It is commonly perceived that treatment with appropriate antibiotics at the early stages of the disease assures successful recovery. However, at the later stages of LD the response to antibiotic treatment may not be successful leading to a persistent (chronic) form of this illness. LD treatment still faces many challenges as the rate of disease progression and the patient’s response to treatment may vary due to individual predispositions, immune system efficacy, and the very effective survival/adaptive strategies of Borrelia sp.
Prevention: You can protect yourself against LD by using insect repellents, tick eliminating kits, and wearing proper clothing when accessing any wooded areas/parks, and afterwards examining yourself and your pets. Always remove any ticks noticed on your skin as quickly as possible and be alert for any signs of a red rash, inflammation, and/or swollen areas on your body. There are no known pharmacological treatments to prevent this disease.
Natural approach: In a continuous search for effective therapy for LD patients and animals, the use of natural, non-toxic treatment still remains an unexplored area. The Cellular Health approach and research strategies are based on simultaneously targeting the genesis of LD (i.e., by eliminating all diverse forms of Borrelia sp.) as well as the symptoms affiliated with it (i.e., inflammation and abnormal levels of body biomarkers, electrolytes, vitamins, microelements, hormones, etc.) through the application of a specific synergistic team of micronutrients (phytobiologicals such as vitamins, nutrients, and phytochemicals). It opens up new possibilities for safe and effective control of LD and bringing hope for millions of LD patients.
1. How Humans can get Lyme disease

The human transmission of Lyme disease starts from ticks. Ticks are small common external insects of the spider family that feed on blood sucked from humans and animals. The tick becomes infected by pulling bacteria called spirochetes that cause Lyme disease, while feeding on the animal carrying this pathogen. The spirochetes then multiply in the tick’s midgut and are ready to be transferred into the next host (such as animals and humans). The animals that most often carry ticks are white-footed field mice, deer, raccoons, opossums, skunks, weasels, foxes, shrews, moles, chipmunks, squirrels, and horses.
Ticks harboring this bacterial pathogen are from genus Ixodes and belong to the five following species: I. daminii, I. scapularis, and I. pacificus (in Northern America) as well as I. ricinus and I. persulcatus (in Europe). The black-legged tick (called deer tick or Ixodes scapularis) spreads the disease in the northeastern, mid-Atlantic, and north-central United States, while the western black-legged tick (or Ixodes pacificus) spreads the disease on the Pacific Coast. In the USA the proportion of ticks reported to harbor Borrelia sp. ranges between 1-100% and in Europe between 0-85%. There are even greater variations among the different counties within the states and from area to area within a county or state.
In nature, ticks undergo sequential transformation from egg, to larva, to nymph, and finally they become an adult form called imago. Although ticks can spread the infection at all development/maturation stages, the most concerning are the nymphs since they are abundant during spring and summer. Nymphs are small (1-2 mm) and their bite is painless, and thus they are very difficult to detect. Ticks can attach to any part of the human or animal body but are often found in the areas most easy to be missed, such as the scalp, the armpits, and groin.
NOTE:
- There is no evidence that Lyme disease is transmitted from person to person, however, Lyme disease developed during pregnancy may lead to infection of the placenta and possible miscarriage.
- Recently some connection between Lyme disease and autism was also brought to light. Moreover, there are reports that spirochetes of Lyme disease can live in blood that is stored for donation, and for that reason donors infected with Lyme disease should not donate blood.
- Pets can also get Lyme disease and bring infected ticks into the home or garden.
- There is yet no trustworthy evidence, that Lyme disease is an air-borne disease, which means it cannot be transmitted through air, food, water, or from the bites of mosquitoes, flies, fleas, or lice.
...hide content
2. How prevalent is Lyme disease
The latest statistics confirm that Lyme disease is the most common vector-borne disease in the USA (with ~30,000 cases annually) and in Europe (with ~65-80,000 cases stated each year). However, these numbers reflect only reported cases, and the real numbers may be even 5-10 times higher due to frequent misdiagnosis of Lyme disease and false-negative results that may occur in as many as 50% of the cases.
Lyme disease has been reported in all continents except Antarctica. In the USA almost all states face a problem. However, most Lyme disease cases (~95%) have occurred in Connecticut, Delaware, Maine, Maryland, Massachusetts, Minnesota, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, Vermont, Virginia, and Wisconsin. Lyme disease has been reported in almost all countries in Europe, especially in the Scandinavian countries, Germany, and Slovenia. For more information please see the map representing Lyme disease presence in the USA and Europe.
...hide content
3. What are the symptoms of Lyme disease
Lyme disease is manifested as an inflammatory disease that can affect many organs in the body. In its early (localized) stage it affects mainly the skin. However in later stages (disseminated and/or chronic) the inflammation can spread to the joints, nervous system and, to a lesser extent, the heart, muscles or other organs.
The symptoms of Lyme disease may suddenly disappear even without any treatment or after a mild treatment. Also, not all symptoms have to always appear in a patient, and different symptoms may appear at different times. Several medical disorders, recognized over the years as separate clinical cases, i.e., acrodermatitis chronica atrophicans (ACA), lymphadenosis benigna cutis (LABC), erythema migrans (EM) and, lymphocytic meningoradiculitis (Bannwarth's syndrome), are currently accepted as the indicators of Lyme disease.
...hide content
4. Stages of Lyme disease progression
There are four recognized stages of Lyme disease:
- Early localized stage (3-30 days post-tick bite). If Lyme disease is not adequately treated at this stage, it will spread from the site of tick’s bite to other parts of the body, generating a plethora of symptoms that may randomly appear and disappear.
Symptoms of early localized stage include:
♦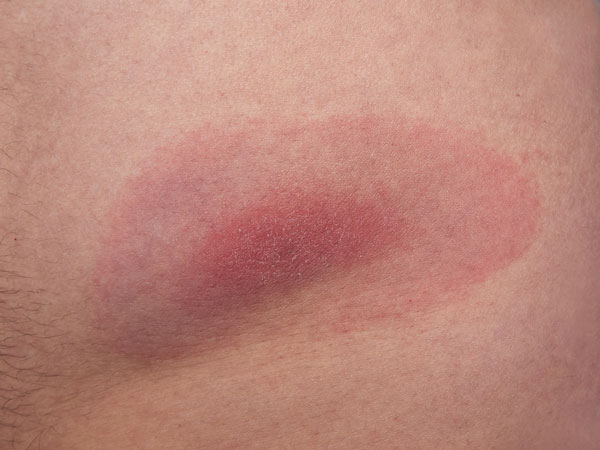 Erythema migrans (EM): A red spot (skin lesion) at the site of the tick bite, which will expand over time forming a bull's eye pattern is the hallmarks of Lyme disease. However, only about 10% of patients develop the appearance of a classic bull’s eye. In some the rash can appear at more than one area of the body. Moreover, ticks very often spread other pathogens that may cause a different type of rash. Also, in some people this small bump or redness at the site of a tick’s bite may disappear over the next 1-2 days.
Erythema migrans (EM): A red spot (skin lesion) at the site of the tick bite, which will expand over time forming a bull's eye pattern is the hallmarks of Lyme disease. However, only about 10% of patients develop the appearance of a classic bull’s eye. In some the rash can appear at more than one area of the body. Moreover, ticks very often spread other pathogens that may cause a different type of rash. Also, in some people this small bump or redness at the site of a tick’s bite may disappear over the next 1-2 days.
♦ Flu-like symptoms: Symptoms include fatigue, chills, fever, headache, muscle and joint aches, swollen lymph nodes, and nausea. It is important to note that these symptoms may vanish without treatment. Some people may get these non-specific symptoms together with the erythema migrans rash, or these general symptoms may be the only sign of infection.
- Early disseminated stage (days to weeks post-tick bite). Lyme disease not adequately treated at this stage can lead to developing a variety of health complications by allowing the pathogens to go into hiding places in the body.
Symptoms of early disseminated stage include:
♦ Additional rashes (erythema migrans) in other places on the body
♦ Fatigue, nausea, diarrhea
♦ Depression, anxiety, mood swings
♦ Cognitive impairment, light/sound sensitivity
♦ Severe headaches and/or neck stiffness due to meningitis
♦ Pain and swelling in the large joints
♦ Shooting pains with or without sleep disturbance
♦ Facial or Bell's palsy (loss of muscle tone on one or both sides of the face)
♦ Heart palpitations and dizziness due to changes in heartbeat
- Late disseminated stage (months to years post-tick bite). At this stage treatment becomes more complicated.
Symptoms indicating late disseminated stage of LD may include:
♦ 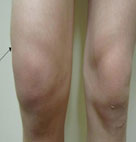 Arthritis: About 60% of patients with untreated Lyme disease develop arthritis (severe joint pain with swelling), usually in the knees, although pain can move from one joint to a second one. It has been pointed out that some HLA markers (HLA-DRB1*0401 and HLA-DRB1*101) are related with host immune responses due to their interaction with the Borrelia burgdorferi OspA protein. Note: arthritis manifests differently than arthralgia (pain, but not swelling).
Arthritis: About 60% of patients with untreated Lyme disease develop arthritis (severe joint pain with swelling), usually in the knees, although pain can move from one joint to a second one. It has been pointed out that some HLA markers (HLA-DRB1*0401 and HLA-DRB1*101) are related with host immune responses due to their interaction with the Borrelia burgdorferi OspA protein. Note: arthritis manifests differently than arthralgia (pain, but not swelling).
♦ Neurological complications: Up to 5% of patients with untreated Lyme disease develop neurological symptoms such as shooting pains, weakness or itching/tingling in the hands and/or feet, short-term memory deficiency, impaired muscle movement, and severe fatigue. Also, heart problems (an irregular heartbeat), and inflammation of the eyes and liver (hepatitis) can appear.
- Persistent (chronic) stage (called PTLDS, Post-Treatment Lyme Disease Syndrome).
Symptoms of chronic/persistent stage may last for decades and may include:
♦ Muscle and/or joint pains
♦ Cognitive defects
♦ Sleep disturbance
♦ Fatigue
These symptoms proceed for months to years after treatment and develop in approximately 10-20% of patients with Lyme disease. They are allegedly caused by an autoimmune response of the organism and cause lasting damage to the host’s tissues. However, the exact cause is unknown. Importantly, studies have shown that continuation of antibiotic therapy does not help and can even be destructive. According to LymeMD (www.lymemd.blogspot.com) and ILADS (International Lyme and Associated Diseases Society) these patients are also facing:
● Changes in mental status
● Neurological abnormalities such as a deviated uvula and or soft palate, decreased sensation on one side of the face, asymmetry of the face, restricted movement of the eyes with extreme lateral gaze, hearing loss, deviation of the tongue with protrusion, Hoffman or Babinsky reflexes, decreased sensation to pinprick of the extremities in a "stocking and glove" pattern, and a loss of vibration sense in the feet compared with the hands
● A low CD57 count
● Low magnesium level
● A Lyme C6 peptide antibody index which exceeds 0.1
● Antibodies to co-infections such as by Babesia sp., Ehrlichia sp., and Bartonella sp.
● Mild elevation of auto-immune disease markers including: RT (rheumatoid factor) and ANA (antinuclear antibodies)
● Low or borderline levels of vitamin B12 and folic acid
● A shift in vitamin D levels characterized by low vitamin 25-Hydroxy Vitamin D (25-OH-VitD) and high vitamin 1,25-Dihydroxyvitamin D (1,25-OH-VitD)
● Abnormal brain MRI showing non-specific white matter disease
● Abnormal brain SPECT scan showing changes in blood flow in the brain
Jarisch-Herxheimer reaction: Approximately 15% of Lyme disease patients have post-treatment reactions (within 24 hours or longer after treatment) called Jarisch-Herxheimer, manifested by elevated temperature, myalgia, and arthralgia, resulting from increased levels of circulating antigens/endotoxins derived from dead spirochetes and latent forms.
...hide content
5. Diagnosis of Lyme disease
Diagnosis of Lyme disease is not easy and is often a challenge for a physician. Currently Lyme disease is diagnosed based on:
♦ History of possible exposure to infected ticks
♦ Symptoms
♦ Laboratory blood tests
Relying only on symptoms is not dependable since they are non-specific and often mimic other medical conditions, because ticks can co-transmit other infections at the same time . Various symptoms (e.g., "auto–immune" responses) may occur if other infections are involved such as Campylobacter (Guillain-Barre syndrome), Chlamydia (Reiter's syndrome), and Strep throat (rheumatic heart disease). In addition, about 7-10% patients may not develop symptoms (called asymptomatic patients).
Commonly performed laboratory tests
♦ ELISA (Enzyme-Linked Immunosorbent Assay, also called enzyme immunoassay or EIA): This test verifies the presence of infection by detecting antibodies produced by the patient’s body against spirochete Borrelia burgdorferi. ELISA test evaluates the body’s response to infection rather than presence of the bacteria. Current ELISA tests (i.e., EIA or IFA) are not sensitive enough for routine screening and may give false-negative results in up to 50% of the cases.
♦ Western blot (or immunoblot): This blood test is usually performed when the ELISA test is positive in order to confirm the diagnosis. Western blot detects antibodies to several proteins of Borrelia burgdorferi. Due to poor sensitivity of the ELISA tests, the Western blot is a better option. However, different laboratories use different methods and validation criteria, and a patient can have a positive test result from one lab and a negative test result from another. Western blot test is more reliable when performed at least a month after a tick’s bite when the infection has already developed, although none of the current tests are 100% accurate:
Other tests
♦ Cultivation: This method allows for direct detection of the presence of bacteria in the body. Identification of Lyme bacteria presence in a patient’s blood is a “gold standard” test done by culturing serum/blood or biopsies taken from a patient. A blood (or tissue) sample is added to a special medium and left for a certain time to allow the bacteria to grow so it can be visually detected. Although this method gives evident proof of infection, it is not routinely performed because Lyme disease bacteria grow very slowly and the results are not available quickly. Also, there are no commercially available culture tests for Lyme disease.
♦ PCR (Polymerase-Chain Reaction): This test detects bacterial DNA in fluids obtained from a patient’s infected joint or cerebrospinal cord fluid (CSF). PCR multiplies specific fragments of the Lyme spirochetes’ DNA. It can detect the bacteria presence, but also gives many false negative results. It is not very effective in diagnosing Borrelia infection in the blood or urine; however, it is useful to indicate chronic Lyme disease. PCR based tests are not frequently performed as they need specific equipment, are costly, and require trained and skilled personnel to perform them.
It is estimated that about 20-30% of patients can have false negative antibody/serological test results, however, some reports quote that this number can reach even 50%. The reason for that is that people with early stages of Lyme disease may not produce enough specific antibodies for several weeks from infection to reach the level detectable by these diagnostic tests. Also, signs and symptoms of Lyme disease are non-specific and often mimic other medical conditions, and ticks can transmit other diseases at the same time. However, delaying treatment by waiting for reliable test results gives the pathogens additional time to disseminate in the body. If early symptoms are undetected, ignored, or treated incorrectly, patients can progress to more advanced stages of Lyme disease with more severe symptoms appearing weeks, months or perhaps even years after a tick’s bite.
Therefore, today LD diagnosis is based primarily on clinical recognition and evaluation of symptoms of Lyme disease in a person who has a high probability of attracting it by living or being in a high risk area. In patients with erythema migrans, diagnosis of early Lyme disease should be made on the basis of symptoms and evidence of a tick bite. As mentioned above, serological tests have poor sensitivity in patients with erythema migrans during acute phase (positive results are in 25-40% of patients). These tests are more accurate in patients with early disseminated neurological and cardiac Lyme disease (positive results are in 80-100% of patients), or with late manifestations of Lyme disease such as arthritis (positive results - nearly 100% patients).
...hide content
6. How Lyme disease can be treated
Contemporary medicine cannot offer an effective cure for Lyme disease. There are two main approaches to management of this disease and the medical community is still debating about which is the best and the final decision has not yet been reached:
♦ The Infectious Disease Society of American (IDSA) recommends two weeks of antibiotic treatment for early Lyme and does not recognize chronic Lyme disease.
♦ The International Lyme and Associated Diseases Society (ILADS) recommends flexible individualized treatment based on patient response.
Conventional treatments
These treatments rely mainly on antibiotics:
♦ Oral antibiotics: Antibiotics commonly used for oral treatment include β-lactam antibiotics such as doxycycline, amoxicillin, or cefuroxime axetil. These are typically recommended for an early form of Lyme disease: usually doxycycline for adults and children over 8, and amoxicillin or cefuroxime for adults, younger children, and pregnant or breast-feeding women, and are prescribed for 14-21 days of administration. Another type are macrolide antibiotics such as Azithromycin, Clarithromycin, Erythromycin that are used if a patient has contradictory conditions to β-lactams.
♦ Intravenous antibiotics: Antibiotics commonly used for intravenous treatment include ceftriaxone, cefotaxime or penicillin. These are typically advised for treatment of late stages of Lyme disease and are administrated for 14-28 days. Intravenous antibiotics are administrated longer and can cause various side effects, including a lower white blood cell count, mild to severe diarrhea, or colonization or infection with other antibiotic resistant organisms unrelated to Lyme bacteria.
♦ No vaccine is currently available for humans.
Alternative approaches
Many desperate patients dissatisfied with a lack of efficacy of prescribed medicines are turning to natural approaches which are generally safe without the severe side effects that are associated with pharmaceutical drugs.
Several plant extracts, oils, enzymes (proteases), vitamins, etc., have been used against Lyme disease, however with mixed results. Their application has mostly been based on the efficacy of these substances against various types of bacteria, not necessarily Borrelia sp. and on targeting symptoms rather than a causative factor of this disease. Moreover, these substances have been largely considered and applied as individual components mimicking the pharmaceutical drug approach. In this case, it is quite likely that, based on an extreme adaptive ability of Borreliaea, a resistance to such treatment can develop. Therefore, they do not assure persistent efficacy due to their limitations in the eradication of the bacteria (as a primary cause of Lyme disease) and their damaging effects on the patient’s organism (as a secondary cause of Lyme disease).
Only a few naturally occurring compounds extracted, from seeds, leaves, bark, roots of plants (e.g., herbs, spices), were researched to date including grape seed extract, teasel root extract, samento extract (cat's claw extract; two species of cat's claw are currently in use such as Uncaria tomentosa and Uncaria guianensis), and banderol extract (plant extract from the bark of the Otoba sp., a tree growing in South America).
Cellular health and micronutrient synergy approach
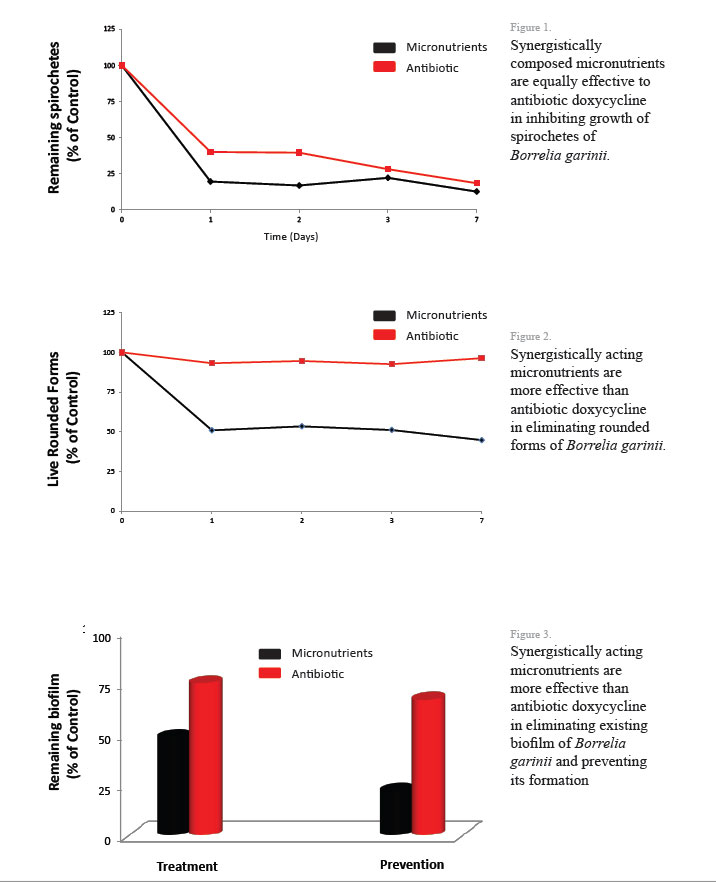
At the Dr. Rath Research Institute we pioneered the nutrient synergy approach in developing new strategies in a natural control of various pathologies, including Borrelia infection.We tested the efficacy of 50 different natural compounds against two species of Borrelia: Borrelia burgdorferi sensu stricto (pathogen causing Lyme disease in the USA) and Borrelia garinii (pathogen causing Lyme disease in Europe). The initial results have been very encouraging. After evaluating the efficacy of these natural components against all known morphological forms of Borrelia (spirochetes, rounded forms and biofilm), we selected specific phytobiologics (phytochemicals and vitamins) which affected all these forms at once. Further, we identified synergistic composition of these micronutrients which displayed higher anti-borreliaea efficacy compared to their individual components or their random combinations. The results indicate that the mixture of these micronutrients are more effective in eliminating Borrelia rounded forms and biofilm than commonly used antibiotic, Doxycycline.
In vivo studies:
We treated healthy (control) and sick mice with our mix for 4 weeks. Samples collected at the end of the experiments were subjected to testing for clinical parameters. We also looked for any adverse effects. We did not notice signs of weakness, aggressiveness, death, obesity, or thirst and this told us that mix is well tolerated and did not cause diabetes, anemia, bleeding, etc. To confirm these observations, we subjected the animals’ blood for pre-clinical evaluation and did not notice differences in basic parameters such as levels of red and white blood cells, hemoglobin, and hematocrit and no hemolysis was present. On closer evaluation we noticed elevated levels of monocytes in only the sick group of animals not treated with mix. This means that the immune system recognized Borrelia as an invader (injected pathogen survived and the diseased developed). We confirmed this result by subjecting the biopsies from different organs of all animal groups to qPCR assay, which is a qualitative and, at the same time, quantitative method allowing for detection of Borrelia DNA. We detected Borrelia DNA in all animals injected and not treated, whereas almost 90% reduction was seen in animals with injected pathogen but treated with our mix. No Borrelia DNA was present in healthy not injected animals. Finally, we looked at inflammation and toxicity by checking appropriate markers. No changes were seen between healthy treated animals and healthy untreated animals, which mean that even in healthy animals mix did not cause any adverse effects. In contrast, increased levels of inflammatory markers were noticed in the sick untreated group but without signs of toxicity. Sick animals treated with mix did not show increased inflammation or toxic effects.
Concerns with conventional Lyme disease treatment
There is a common perception that patients treated with antibiotics at the early stages of Lyme disease recover rapidly and completely, and that the later disease stages can also be treated effectively, although recovery is slower. However, in reality, the rate of disease progression and individual response to these treatments vary. Some patients face no signs of disease and others have symptoms that remain for months or even years following the treatment. In addition, there are many concerns with a long-term antibiotic treatment and, since its effectiveness has been neither observed nor proven, it is generally not recommended. In addition, it can lead to antibiotic resistance. Unfortunately, the number of newly infected people is increasing and many those who already had Lyme disease are relapsing and developing persistent (chronic) symptoms.
Reoccurrence of disease is mainly attributed to inadequate prevention, ineffective therapy, and development of bacterial resistance. On average approximately 10-20% (even up to 50%) of patients who followed appropriate antibiotic treatment may face significant, persistent or recurrent symptoms of Lyme disease such as joint and/or muscle aches/pains and fatigue which can last for many months or even years lowering their quality of life and making subsequent treatment more complicated. At the beginning of oral or IV antibiotic treatment, these patients may have experienced a slow health improvement; however, prolonged antibiotic treatment (longer than 4-6 weeks) is usually not recommended due to side effects and serious health complications. The cause of these persisting symptoms is unknown
Animal studies indicate that antibiotic treatment in dogs may not always lead to complete elimination of spirochetes. Even more, after 4 weeks of antibiotic therapy in mice there was an induced re-activation of Borrelia burgdorferi from its latent forms causing spirochetemia. There is also scientific evidence indicating that after a long-term administration of antibiotics to mice the infective spirochetes can persist in mice without inducing signs of a disease, but the authors of this study project this attenuated population of spirochetes will eventually die or be killed by host defenses.
...hide content
7. What is known about the bacteria which cause Lyme disease
A. Various Borrelia species
The causative pathogen of Lyme disease is a bacterium of genus Borrelia, which has at least 37 known species. Twelve of them are Lyme-related and the number of genomic strains is unknown. All Borrelia sp. are host-dependent, invasive, micro-aerophilic and slow-growing and this is the primary reason of the complications with diagnosing Lyme disease. Until recently, only three genetic species were thought to cause Lyme disease such as:
♦ B. burgdorferisensu stricto: the predominant species in North America, but also present in Europe
♦ B. afzelii and B. garinii: both predominant in Eurasia
In addition, there are 10 more bacteria species emerging, such as:
♦ B. mayonii isolated in the U.S.
♦ B. valaisiana isolated throughout Europe as well as East Asia
♦ B. lusitaniae present in Europe (especially Portugal), North Africa and Asia
♦ B. bissettii identified in the U.S. and Europe
♦ B. spielmanii present in Europe
♦ B. burgdorferi sensu lato: B. japonica, B. tanukii and B. turdae (in Japan); B. sinica (in China); and B. andersonii (in the USA)
♦ B. miyamotoi spirochete, related to the relapsing fever group of spirochetes suspected of causing illness in Japan. Spirochetes similar to B. miyamotoi have recently been found in ticks of both species such as Ixodes ricinus (in Sweden) and Ixodes scapularis (in the USA)
Additionally, B. lonestari transmitted by ticks of species Amblyomma americanum (lone star tick) in the U.S. is suspected of causing southern tick-associated rash illness (STARI), also known as Masters disease after Edwin Jordan Masters. This illness clinically resembles Lyme disease but currently there is no diagnostic test available to identify it and no official treatment exists as well (although it is usually based on prescribed antibiotics).
Although clinical symptoms after infection with different species may vary, common indicators have been specified. Except for B. recurrentis (which causes louse-borne relapsing fever and is transmitted by the human body louse), all known species are believed to be transmitted by ticks.
B. Why it is difficult to eradicate Borrelia once the infection occurs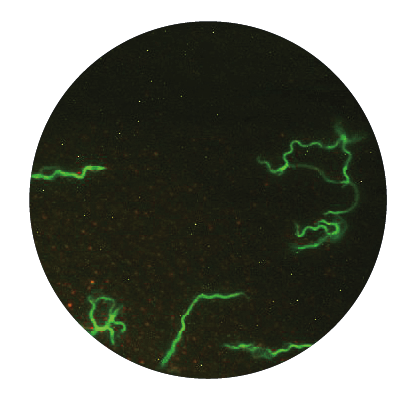
Borrelia sp. exists in three morphological forms, which allow them to withstand and survive changing and even hostile environments:
1. The vegetative (active) form is a spiral-shaped form called spirochete. This form has a unique characteristic, namely they possess flagella allowing them to be motile and survive viscous conditions and they can even enter into tissue or cells causing intracellular infection. When these bacteria feel threatened, e.g., by starvation, changes in temperature and/or pH, exposure to antibiotics, etc., they quickly adapt to new situations by changing into so called latent forms.
2. Latent (atypical) forms of this bacterium include rounded forms and biofilm. These forms allow the bacteria to survive any hostile condition and become active again after these conditions cease. This is due to their internal ability of genetic adaptation followed by phenotypic changes in the spirochetes. The bacteria become structurally and metabolically different transforming to new, dormant forms. The presence of these atypical forms of Borrelia and their extreme adaptation to environmental changes may be the reason why the spirochete can survive for years or even for decades in the host’s body.
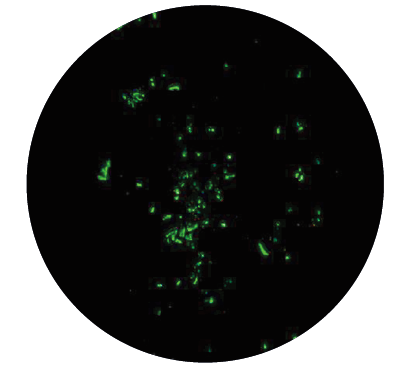
♦ Rounded forms (bodies) are living forms which undergo shape changes facilitated by unique proteins produced in bacterial genome. They have a low metabolic ra te, they are not motile but may be transmissible and can induce infection independently of spirochetes presence. The finding that energy is required for the spiral form to convert to latent form is another argument confirming that these altered forms have a survival function, and are not degeneration products. The spirochetes can transform to latent rounded forms of different appearance, such as cysts (round bodies), granular forms (dot-like spirochetes, pearl of strain) or so called CWD forms (spheroplasts, L-forms, bleb-like spirochetes):
● Granular forms disintegrate into particles called granules that are liberated from the spirochete body by budding and extrusion
● CWD (Cell Deficient Wall) forms do not have a cell wall (there is little information available about them)
● Cysts are morphologically diverse. Cysts may contain more than a single spirochete and their division is independent of the division of the spiral forms inside the cyst. Several studies have shown that Borrelia sp. can convert from the spirochete to the cyst when hostile conditions are present and revert back to the spirochete form when conditions are safe for them again. All rounded forms may not be eradicated by short-term antibiotic therapy (mice inoculated intra-peritoneally with cysts produce motile spirochetes generating an infection in the animal).
♦  Biofilm is an organized structure formed by many, if not all, bacteria. It contains a thin layered bacteria attached to host tissues, to other bacteria, or to non-living matrices or medical devices. The bacteria is embedded or covered by a self-produced matrix of extracellular substance composed of complex polysaccharides, proteins, and nucleic acids. Biofilm generated by Borrelia shields them from antibiotics and the immune system components. It can also house other microorganisms, e.g., Mycoplasma and vice versa Borrelia can hide in other microbial's biofilm.
Biofilm is an organized structure formed by many, if not all, bacteria. It contains a thin layered bacteria attached to host tissues, to other bacteria, or to non-living matrices or medical devices. The bacteria is embedded or covered by a self-produced matrix of extracellular substance composed of complex polysaccharides, proteins, and nucleic acids. Biofilm generated by Borrelia shields them from antibiotics and the immune system components. It can also house other microorganisms, e.g., Mycoplasma and vice versa Borrelia can hide in other microbial's biofilm.
Within the biofilm, Borrelia is capable of communicating with its own species or other bacteria and develops various aggressive measures against an animal or human host. Borrelia can also receive and exchange genetic information such as drug resistant genes. This is one of the reasons why even many years of administration of antibiotics cannot rid the infection. Although, immune cells (phagocytes) can recognize and attach to the biofilm they are unable to consume it. That is why a biofilm is considered to facilitate a persistent infection. Recent studies point out that Borrelia sp. contained within the biofilm are much more difficult to eliminate being 1000 times more resistant to antibiotics than the spirochetes. Furthermore, Borrelia may exist as either biofilms attached to the surface or as a planktonic-like free floating biofilm structure in living (such as blood) and/or non-living environments (such as broth). Biofilm can be either composed of only one morphological form of bacteria (homogeneous biofilm) or contain a variety of forms (heterogeneous biofilm).
...hide content
8. Pathogenesis of Lyme disease
A. Survival and adaptive strategies of Borrelia sp.
After infection, Borrelia may persist in humans and animals for months or even years and, as some studies have shown, regardless of whether the disease was or was not treated with antibiotics. Various survival strategies of Borrelia have been postulated and scientifically proven showing how the spirochetes can persist in their host.
They include:
♦ Physical location or relocation in body tissues to sites less accessible to the immune system and antibiotics
♦ Use of the host's enzymatic system such as plasmin and/or proteases (e.g., MMPs) to destroy collagen and connective tissue allowing the bacteria to move in the tissue, penetrate blood vessels and cross a blood–brain barrier
♦ Invading a variety of cells and “hiding” inside of them during human infection; Borrelia may be able to evade the immune system this way and to some degree be protected against antibiotics (coiling phagocytosis)
♦ Altering their spirochete forms by converting to various rounded forms (cysts, granules, spheroplasts) and biofilm
♦ High antigenic variation and changing gene expression: Borrelia sp. have the ability to modulate its surface protein expression in response to environmental threats thereby evading the immune system and establishing a chronic infection (e.g., the outer envelope of the cyst has no OspA protein, therefore all antibodies the body produces against the spirochetes are not effective to fight latent rounded forms)
♦ Immune system suppression: complement inhibition, induction of anti-inflammatory cytokines, and the formation of immune complexes (their presence has been shown to be involved in false-negative serological tests of blood and cerebrospinal fluid)
♦ Resistance against phagocytes and lymphocytes
B. Molecular mechanisms involved in Lyme disease progression
1. Colonization of the bacteria (multiplication, spreading, inducing an inflammatory response)
♦ Genes involved: ↓ OspA -> ↑ OspC/OspE-F (Erps), Rev, DbpA-B, VraA, P47 (BBK32), BBF01, 41 kDa -> Vmps (Vlps/Vsps)
2. Entering the bloodstream and colonizing tissues
♦ Penetration of blood vessels and homing
♦ Attachment to tissue cell surfaces and matrix glycosaminoglycans (integrins, selectins, VCAM and ICAM) as well as ECM proteins (extracellular matrix proteins)
♦ Activation of plasmin and induction of host proteases to facilitate tissue dissemination and/or inflammation
3. Persistence
♦ The vls antigenic variation system (down-regulation of highly antigenic surface proteins - no OspA-F proteins)
♦ Complement-regulator acquiring surface proteins (CRASPs)
♦ Anti-OspA Ab presence, self-reacting T lymphocytes existence, MHC class I and II polymorphism
♦ Coiling phagocytosis (homing of pathogen inside the cells)
♦ Anti-inflammatory cytokines
...hide content
9. How can I protect myself against Lyme disease
It is important to take precautions when planning any trip to wooded areas or grasslands and yards where Lyme disease prevails, since these are the preferred dwellings of ticks. Ticks do not handle sunny lawns because they dry out quickly and die. The peak of infection with Lyme disease has been observed in late spring, summer, and in early fall when juvenile ticks are starting to feed. Being bitten by deer ticks during winter months is an exception.
To prevent tick bites:
♦ Wear long sleeves and tightly laced bright clothes that are tucked into pants and boots when walking throughout these risky areas
♦ Routinely check yourself, your family and pets and all clothing for ticks, especially after outdoor trips
♦ Shower and shampoo your hair and wash and tumble dry clothes in a dryer on high heat for an hour to kill remaining ticks
♦ People often apply tick repellents with DEET (N, N-diethyl-m-toluamide) to clothes, shoes and socks before going out. The caution with using all these chemical repellents is that they can cause serious side effects, particularly when used frequently or in high concentrations. They should not be used on infants and children as they are especially at risk for adverse reactions. In addition, chronic exposure to DEET may induce insomnia, cognitive impairments, etc. Although DEET has been approved for use in children, caution is warranted. Another repellent called permethrin (containing 0.5% of permethrin) is designed to be placed on clothing and can be used alone or in combination with DEET. Permethrin belongs to the family of synthetic chemicals functioning as neurotoxins. It is dangerous especially to fish, honey bees and cats. Other repellents registered by the Environmental Protection Agency (EPA) may be found at http://cfpub.epa.gov/oppref/insect/.
♦ Keep grass cut short in your yard/garden
...hide content
10. Additional information
1. Research articles
Our research group
- Phytochemicals and micronutrients and LD
- Phytochemicals and micronutrients and LD-review
- Phytochemicals and micronutrients with doxycycline and LD
- Phytochemicals and micronutrients interactions
- Phytochemicals and micronutrients as a one composition in vitro
- Phytochemicals and micronutrients as a one composition in vivo
Other research groups
2. Webinars
- Webinar (English): "Micronutrients against Lyme Disease"
- Presentation (English): "Lyme Disease-Beyond Antibiotics"
- Presentation (English): "Lyme Disease - Immune system: friend or foe?”
- Webinar (Polish): "Borelioza - Rosnacy Problem Zdrowotny"
- Webinar (Polish): "Borelioza – Oprócz Antybiotyków"
- Webinar (Polish): “Borelioza - Układ immunologiczny: przyjaciel czy wróg?”
3. Guidelines
- ILADS : Treatment Guidelines
- IDSA: Treatment Guidelines
- Dr Rath Research Institute: Treatment Guidelines (English, Polish, German)
...hide content
- Our research group
- Phytochemicals and micronutrients and LD
- Phytochemicals and micronutrients and LD-review
- Phytochemicals and micronutrients with doxycycline and LD
- Phytochemicals and micronutrients interactions
- Phytochemicals and micronutrients as a one composition in vitro
- Phytochemicals and micronutrients as a one composition in vivo
- Other research groups