Effective and safe global public health strategy to fight the COVID-19 pandemic: Specific micronutrient composition inhibits Coronavirus cell-entry receptor (ACE2) expression
Ivanov V, Ivanova S, Niedzwiecki A, Rath M
Dr. Rath Research Institute, San Jose, CA
SUMMARY
Optimum micronutrient intake is the only scientifically proven way to improve general immune resistance against infections, a fact documented in every leading textbook of biology. This study provides scientific evidence that, in addition, specific micronutrient compositions are powerful tools in the fight against the COVID-19 pandemic.
Both, SARS-CoV-2 – the virus that causes the current pandemic – and other coronaviruses enter body cells via a specific receptor, the Angiotensin-Converting-Enzyme 2 (ACE2). The ACE2 receptor is expressed by many cell types, including lung epithelial cells as well as endothelial cells of the vascular system.
Based on our earlier research that demonstrated that specific micronutrients can block several mechanisms of viral infections, we tested the efficacy of these natural compounds in suppressing the expression of the ACE2 receptor on human endothelial cells and small airway epithelial cells.
Our results show that a micronutrient composition comprising vitamin C as well as certain amino acids, polyphenols, and trace elements is able to suppress this viral ‘entry door’ into the body under both normal and inflammatory conditions, which are associated with infections.
Thus, vitamin-rich nutrition and micronutrient supplementation should be implemented as effective, safe and affordable public health strategies to fight the COVID-19 pandemic and help prevent future outbreaks. Optimizing the micronutrient status of the entire population should form the basis for any global strategy to help prevent future pandemics across the world, including the developing nations.
INTRODUCTION
The coronavirus (COVID-19) pandemic: The scope of the problem
The Corona Virus Disease 2019 (COVID-19) pandemic is one of the greatest threats to mankind in modern history. In many countries of the world, the pandemic is still ongoing posing a significant health threat to millions of people living today. Meanwhile the life of future generations is already being compromised by the staggering economic costs of coping with this pandemic.
Moreover, while humanity is still struggling with the current coronavirus pandemic, experts are already warning of a recurrence in the form of a ‘second wave’ and of future pandemics with yet unknown pathogens (Wang LF 2020). Considering the cumulative health and economic damages such future events would predictably inflict on mankind, it is imperative that we quickly develop public health strategies that effectively reduce the risk of future pandemics.
The precondition for a pandemic
The outbreak of a pandemic is dependent on two main factors: the aggressiveness of a virus/pathogen and the strength of our immune system. A pandemic develops if the immune system of the world’s population is compromised and unable to resist an aggressive virus or other pathogen. The only scientifically proven way for humans to strengthen their immune system in general and better resist a multitude of infective agents, is through optimum nutrition, in particular the intake of micronutrients – vitamins, minerals and other micronutrients – in the form of a vitamin-rich diet or nutritional supplements.
Micronutrient deficiency and the coronavirus pandemic
The current coronavirus infection could only have turned into a pandemic, because it was ‘feeding’ on a widespread pre-existing pandemic: a chronic deficiency of micronutrients affecting hundreds of millions of people worldwide.
All ‘hotspots’, where the current pandemic spread particularly fast confirmed this conclusion. These hotspots included developing countries, economically weak industrialized countries, large cities and metropoles, senior homes, and similar institutions – and even the crews on military ships remaining at sea for extended periods. All these hotspots were characterized by either undernutrition, malnutrition or consumption of micronutrient-poor processed foods. The common denominator linking them is a chronic deficiency in dietary micronutrients.
Key mechanism of coronavirus infection
The COVID-19 pandemic is caused by a virus designated SARS-CoV-2, a member of a group of viruses that cause Severe Acute Respiratory Syndrome, hence the name SARS. This infectious disease started as a regional epidemic in China and rapidly spread to become a global pandemic (Poon 2020, Wang 2020, Zhu 2020).
The sole known cellular ‘entry door’ by which coronaviruses can infect body cells is the Angiotensin-Converting Enzyme II receptor (ACE2) (Lan 2020, Li W 2003, Hoffman 2005, Yan 2020, Zhou 2020). This cellular port is shared by the virus causing COVID-19 along with other coronaviruses that had triggered earlier pandemics (Correa-Giron 2020, Wit 2016).
ACE2 is an integral membrane protein present on many types of cells throughout the human body with particularly strong expression in the pulmonary, cardiovascular, gastrointestinal, and renal systems. Among the cell types expressing ACE2, vascular endothelial cells and lung alveolar cells have been particularly well studied. ACE2-expressing cells may act as target cells and their distribution in the human body may indicate the potential infection routes of the SARS virus (Hamming 2004, Wan 2020).
COVID 19 – a systemic disease
At the beginning of the current pandemic COVID-19 was thought to be primarily an infectious disease affecting the lungs (pulmonary system) of the patients. Soon, however, it became clear that the new coronavirus also directly affected the endothelial cells, the inner cell lining of the blood vessel wall (Varga 2020). When compared to the influenza virus, the cause of the common flu, coronaviruses were frequently found inside the endothelial cells and COVID-19 was associated with a nine times higher frequency of microscopic blood clots (microthrombi) along the blood vessel walls (Ackermann 2020).
A generalized inflammation of the endothelial cells along the blood vessel system (endotheliitis) is today considered one of the reasons why COVID-19 can affect essentially all organs (Pons 2020, Mosleh 2020), including the heart (Bavishi, 2020), brain (Koralnik 2020) and other organs. Thus, any effective therapy against COVID-19 must not only efficiently protect the lung but also the vascular system.
Limitations of vaccines
Currently, the attention of the world is focused on finding a vaccine against the COVID-19 pandemic in the hope that this vaccine may terminate not just the current pandemic but also provide some protection against other pandemics. This is, of course, not the case. Even if a potential vaccine would turn out to be effective against COVID-19 now, it could only be effective against this specific virus, and this virus alone. Limiting global health strategies to a COVID-19 vaccine, inevitably leaves humanity unprotected against a multitude of potential future pandemics.
The compelling need for long-term global health strategies
It is evident that there is a great urgency for effective global health strategies that go beyond addressing the current pandemic. These long-term and global health strategies should fulfil the following criteria: 1. Effectiveness against the current pandemic. 2. Effectiveness in strengthening the immune system of the world’s population to help prevent future pandemics – including those caused by yet unknown infective agents. 3. Safety and affordability, so that people around the world can take advantage of it; this is particularly important since any global strategy to fight a pandemic can only be successful if it works for the poorest members of humanity.
Our study rationale
Over the years we have successfully tested specific micronutrients as natural inhibitors of viral infections and identified common biological targets for these natural compounds – independent of specific viral types. Our results showed that vitamin C, especially in combination with other natural compounds such as lysine, green tea extract, quercetin and other micronutrients, could affect key mechanisms associated with infection of human influenza virus H1N1 (Jariwalla 2007), bird flu H5N1 (Deryabin 2008), avian flu H9N2 in vitro and in vivo (Barbour 2009) as well as HIV (Jariwalla 2010). These micronutrients were effective in inhibiting viral infectivity, multiplication, spread and could protect infected tissues from infection-related damage. Moreover, these natural components had better efficacy in protecting for example bird flu virus infected cells than antiviral drugs such as Tamiflu and Amantadine (Deryabin 2008).
We applied this knowledge to the current pandemic and investigated the efficacy of micronutrients in suppressing the cellular expression of ACE2 receptors – used by the coronavirus for cellular entry – in lung epithelial and vascular endothelial cells.
MATERIAL AND METHODS
Reagents
All reagents were supplied by Sigma/Millipore if not indicated otherwise.
Cell cultures
Human Small Airways Epithelial Cells (SAEC, purchased from ATCC) were cultured in Airways Epithelial Cells growth medium (ATCC) in plastic flasks at 37oC and 5% CO2. For the experiment SAEC, passage 5-7, were plated to collagen-covered 96 well plastic plates (Corning) in 100 μL growth medium and were grown to confluent layer for 4-7 days.
Human Aortic Endothelial Cells (HAEC, purchased from Lonza) were cultured in EGM-2 growth medium (Lonza) in plastic flasks at 37oC and 5% CO2. For the experiment cells at 5-7 passages were plated to collagen-covered 96 well plastic plates (Corning) in 100 μL EGM-2 medium and were grown to confluent layer for 3-5 days.
Cell supplementation
The micronutrient combination used was developed at the Dr. Rath Research Institute (San Jose, Ca). The mixture dissolved in 0.1N HCl according to US Pharmacopeia protocol (USP 2040) was designated as a stock solution. For the experiments, cells were supplemented with indicated doses of the supplements in 100 μL/well cell growth medium for 3-7 day. Supplement working concentrations were expressed as millionth parts of a stock concentration per ml (mpsc/mL). The nutrient composition and dosages used in the experiments are presented in Table 1. The inflammation process in SAEC cells was induced by co-incubation with 10 ng/mL human TNF alpha or 100 ng/mL human Interleukin 6 (Sigma).
ACE-2 ELISA assay
Culture plate wells were washed twice with phosphate buffered saline (PBS) and fixed with 3% formaldehyde/0.5% Triton X100/PBS solution for 1h at 4oC, then washed four times with PBS. 200 μL of 1% bovine serum albumin BSA, Sigma) in PBS was added and plate was incubated at 4oC overnight. Rabbit polyclonal anti ACE-2 antibodies (Sigma) were added to 100 μL 1%BSA/PBS for 1.5 h incubation at room temperature (RT). After three wash cycles with 0.1%BSA/PBS wells were supplied with 100 μL anti-rabbit IgG antibodies conjugated with horse radish peroxidase (HRP, Sigma) for 1h at RT. After three wash cycles with 0.1%BSA/PBS the HRP activity retained was determined by incubation with 100 μL TMB substrate solution (Sigma) for 20 min at RT, followed by the addition of 50 μL of 1N H2SO4 and optical density measurement at 450 nm with micro plate reader (Molecular Devices). Results are expressed as a percentage of experimental addition-free control (mean +/- SD, n=6). Non-specific control (wells incubated without anti ACE2 antibodies) mean value (n=6) was subtracted from all sample values.
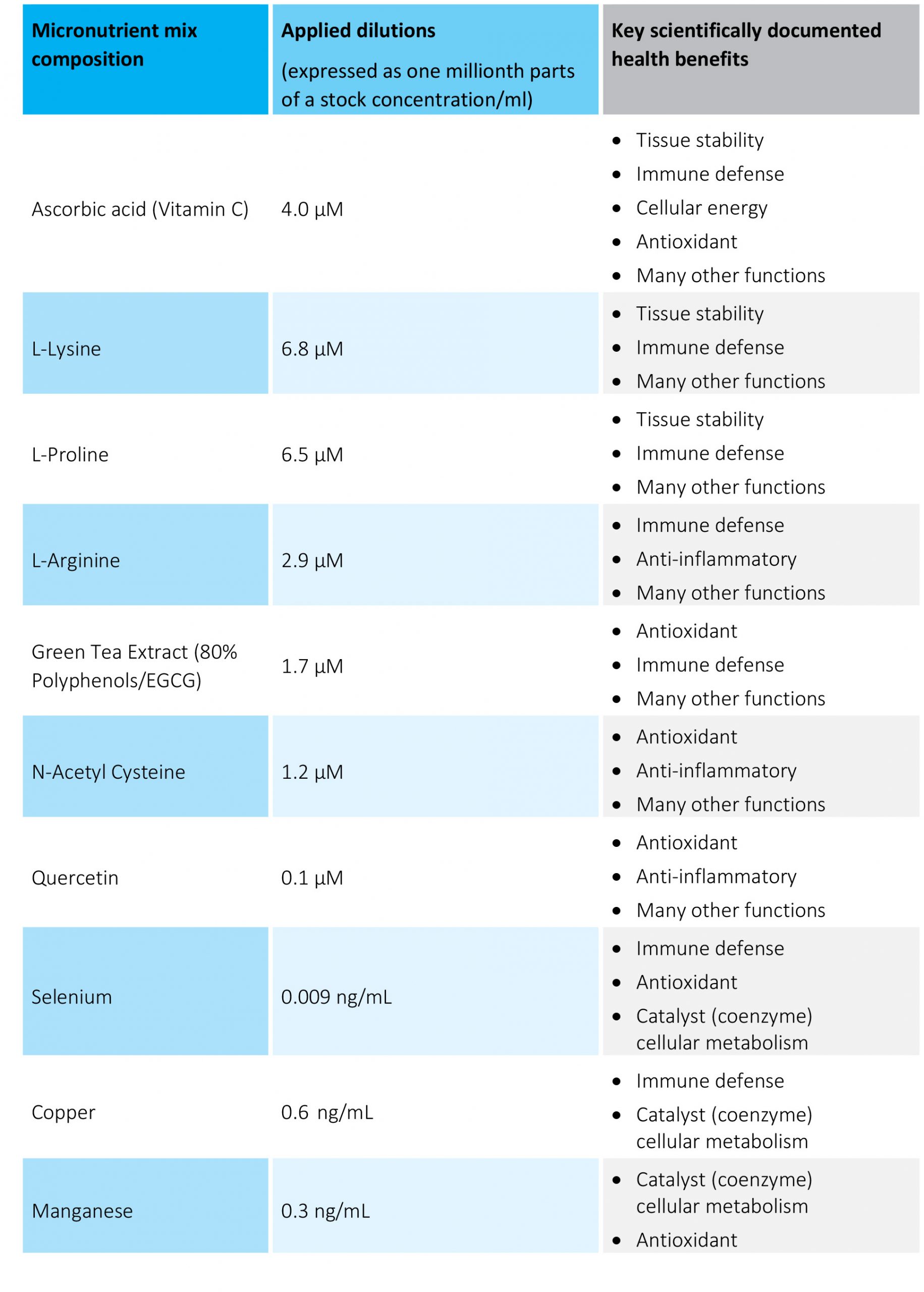 Table 1. The micronutrient composition and concentrations tested.
Table 1. The micronutrient composition and concentrations tested.
RESULTS
Micronutrient composition decreases expression of ACE2 receptor
The effects of the tested micronutrient composition on cellular expression of ACE2 are presented in Figure 1A for human aortic endothelial cells [HAoEC) and Figure 1B for human lung cells (small alveolar epithelial cells, SAEC).
In each cell type the micronutrient composition was able to lower the expression of these viral ‘cell-entry doors’ in a concentration dependent manner. The decrease in ACE2 expression for the highest micronutrient concentration tested was 50% in endothelial cells and 41 % in small airway epithelial cells.
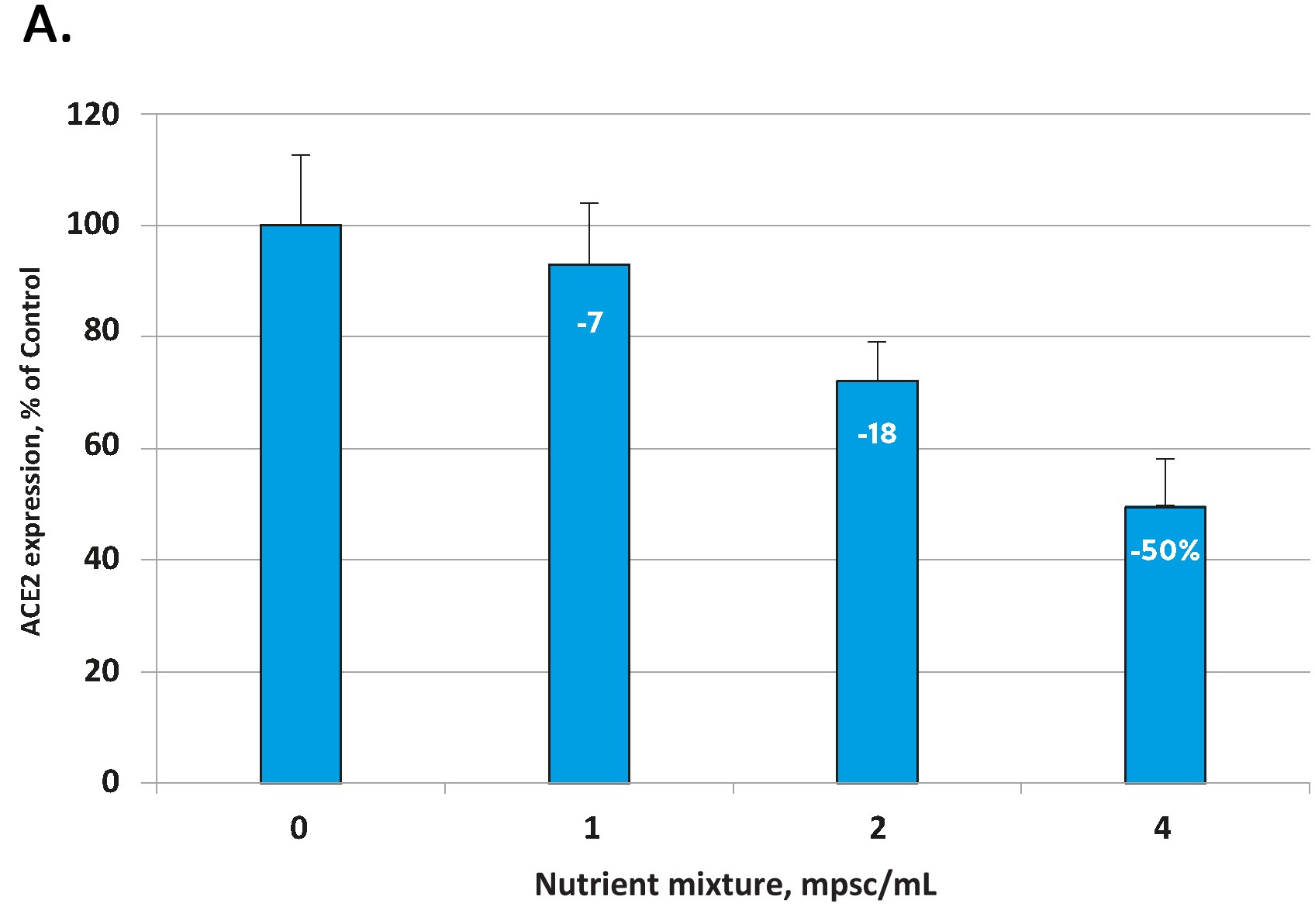
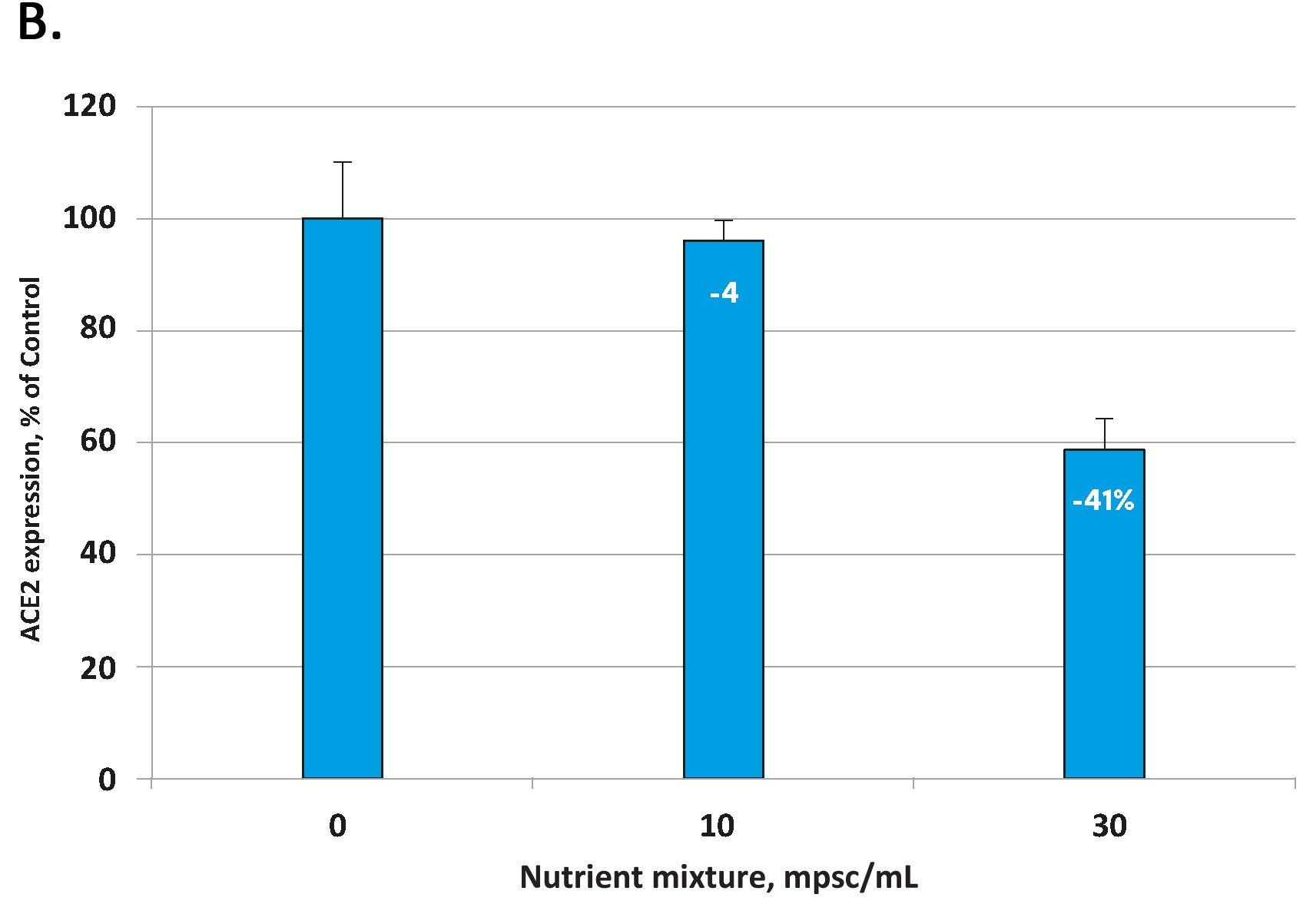
Fig 1. Effect of the tested micronutrient composition on ACE2 expression in human aortic endothelial cells (A) and small airway epithelial cells (B). Increasing concentrations of a specific combination of micronutrients can inhibit the expression of the ACE2 receptor and in human aortic endothelial cells by 50% (A) and in human small airways epithelial cells by up to 41% (B).
Micronutrient inhibit ACE2 receptor expression under inflammatory conditions
Every infection, including COVID-19 is accompanied by inflammation. Inflammatory processes are essentially mediated by biological signal molecules called cytokines. COVID-19 infections are associated with an increase in various inflammatory cytokines including tumor necrosis factor alpha (TNF-α). TNFα has been shown to play a pivotal role in orchestrating the cytokine cascade and has been described as a “master-regulator” of inflammatory cytokine production in many inflammatory diseases.
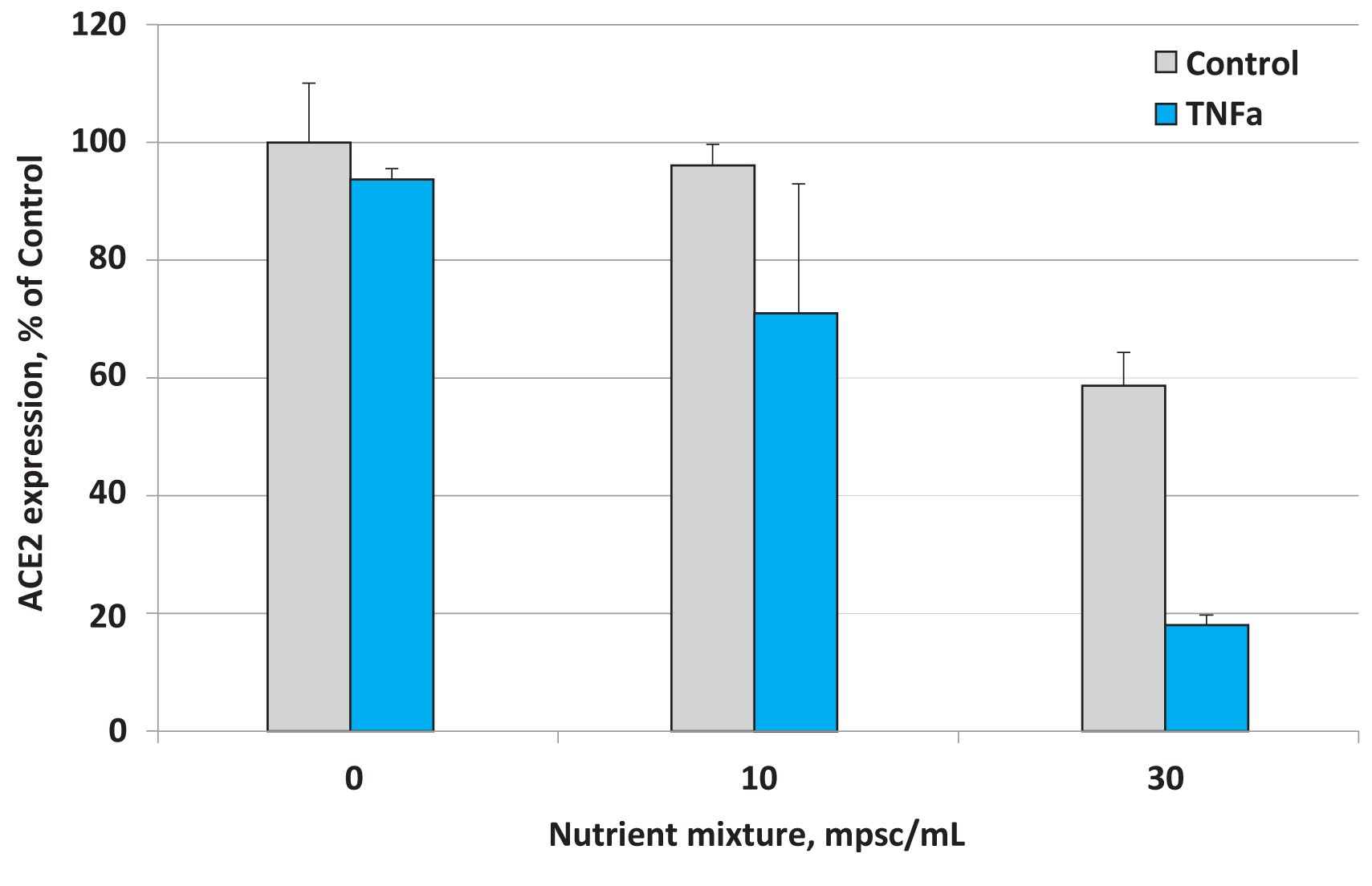 Fig 2. Effects of nutrient composition on ACE2 expression in small airway epithelial cells in the presence of the inflammatory cytokine TNF- α (10 ng/ml)
Fig 2. Effects of nutrient composition on ACE2 expression in small airway epithelial cells in the presence of the inflammatory cytokine TNF- α (10 ng/ml)
The results presented in Figure 2 show that under normal cell culture conditions the micronutrient mixture applied at 30 mpsc inhibited ACE2 expression by 41%. This inhibitory effect, however, was more pronounced in the presence of TNF- α and resulted in lowering ACE2 expression by 81%. This means that the inhibitory effect of this micronutrient composition was significantly enhanced in lung epithelial cells subjected to inflammatory cytokines associated with viral and other infections.
Significance of micronutrient synergy in the control of ACE2 expression
In order to validate the effect of the tested micronutrient composition, we also evaluated the efficacy of its individual micronutrient compounds on suppressing the production of the ACE2 receptor in small airways epithelial cells.
The results presented in Figure 3 show that all individual components, i.e. ascorbic acid (vitamin C), plant polyphenol epigallocatechin gallate(EGCG), quercetin, N-acetylcysteine as precursor of the biological antioxidant glutathione, as well as the natural amino acids lysine and proline were able to lower the expression of the ACE 2 receptor to a varying degree.
It is, however, important to point out that effective dosages of these different natural compounds required to lower the cellular expression of the ACE2 receptor individually, have to be significantly higher compared to the amounts of the same natural compounds used as part of the tested micronutrient combination.
Thus, the micronutrient composition described in this publication serves as an example of ‘synergy’, an important principle of biology. Synergy describes specific interactions of biological compounds – here micronutrients – working together to produce an effect that is not achievable from either nutrient used alone at a specific dose. It achieves a maximum biological effect through regulating cellular metabolism and does not require large amounts of singular bioactive compounds.
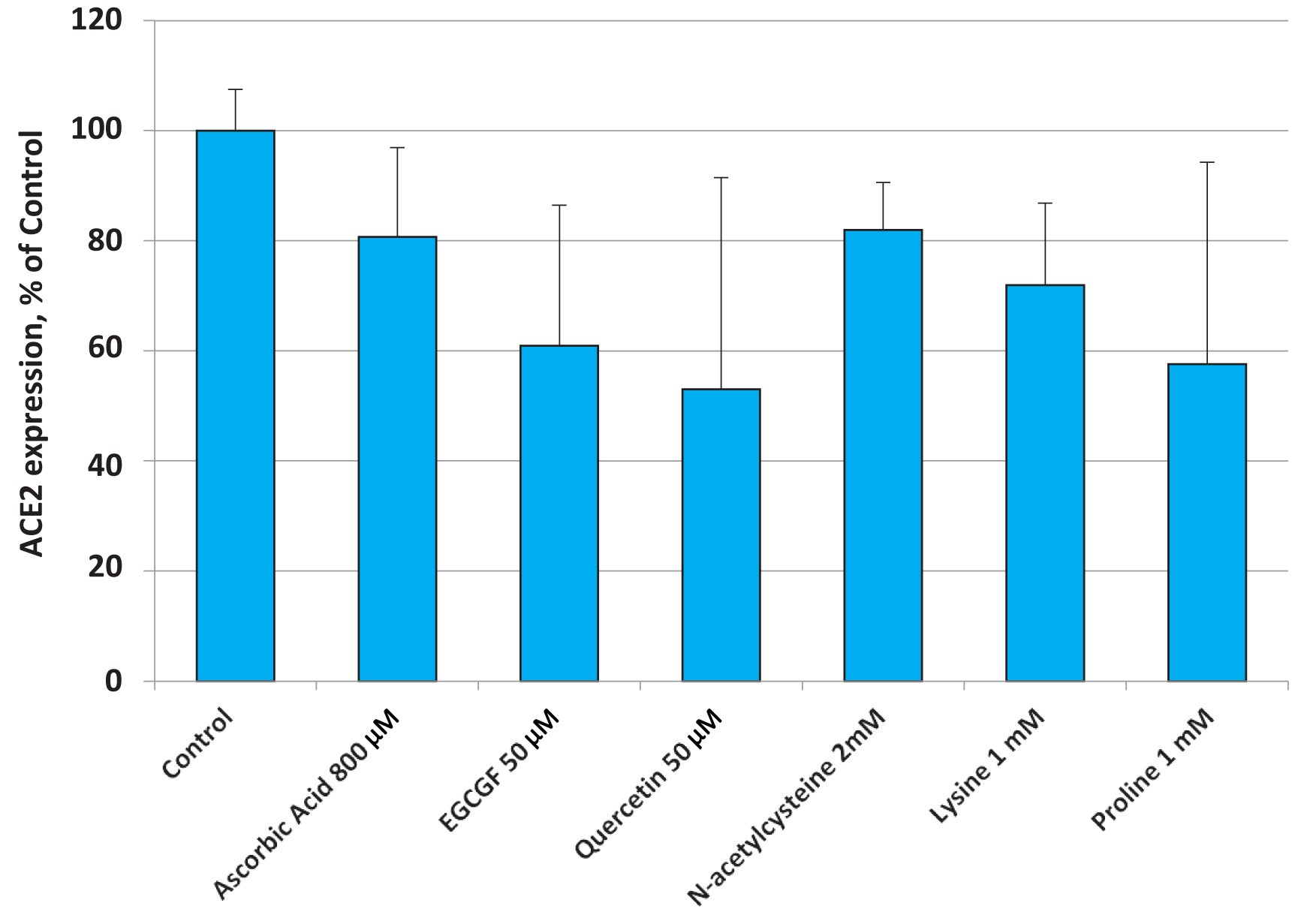 Fig. 3. Changes in ACE2 expression in small airway epithelial cells (SAEC) in the presence of various micronutrients applied at higher concentrations than the ones present in the nutrient mixture.
Fig. 3. Changes in ACE2 expression in small airway epithelial cells (SAEC) in the presence of various micronutrients applied at higher concentrations than the ones present in the nutrient mixture.
DISCUSSION
A novel approach to the control of coronavirus pandemics
The ACE2 receptor is the decisive structure on human body cells mediating the binding and cell entry of coronaviruses including SARS-CoV-2, the cause of the COVID-19 pandemic. Our studies prove that a specific composition of micronutrients can substantially decrease the expression of this ‘cell-entry door’ in human lung (alveolar epithelial) cells, as well as in vascular (endothelial) cells.
Moreover, since all known coronaviruses use the ACE2 receptor for invading human body cells, the findings presented here are relevant to developing public health strategies against the current pandemic. Moreover, they also promise to significantly reduce the risk of pandemics caused by other coronaviruses such as Severe Acute Respiratory Syndrome Coronavirus 1 (SARS-CoV-1), the Middle East Respiratory Syndrome-Related Coronavirus (MERS-CoV), as well as future pandemics with newly emerging coronavirus mutations.
The efficacy of the micronutrient composition proven here to significantly lower the expression of the virus-binding ACE2 receptor could potentially be further enhanced by identifying additional natural compounds that would complement this composition.
Our study confirms earlier observational reports where high-dose intravenous vitamin C or other individual vitamins were successfully used in the clinical treatment of patients affected by COVID-19 (Shanghai Medical Association, 2020). However, thus far scientific studies establishing the mechanism of action of such micronutrients against the coronavirus have been scarce or absent. This lack of scientific data could also explain why micronutrients have been largely absent from the recommendations of governments and international organizations in the battle against the current pandemic. The results presented in this publication should help to close this gap.
To the best of our knowledge, this is the first report providing scientific proof of the efficacy of a composition of natural compounds inhibiting the key entry mechanism of the coronavirus into human body cells.
Micronutrient synergy as key
It is noteworthy, that each component of this micronutrient composition is – individually – also able to down-regulate the expression of ACE2 receptors in human cells. The principle of nutrient synergy applied in our research achieves the desired cellular effect with much lower micronutrient concentrations compared to that achieved when they are used individually. Obtaining higher concentrations of individual micronutrients in human plasma would generally require intravenous delivery, while the nutrient concentration in the composition tested is achievable by optimum oral micronutrient supplementation.
Addressing COVID-19 as a systemic disease with a focus on the pulmonary and vascular system
In addition to the lung, the COVID-19 infection also affects the cardiovascular system of infected patients, because the SARS-CoV has a high affinity for infecting vascular endothelial cells. This fact contributes to the particular aggressiveness of the current coronavirus pandemic (Ackermann, 2020, Pons 2020, Mosleh 2020) and its widespread damage to other organs.
It is, therefore, of the utmost importance to document that the micronutrient combination we have developed is not only able to downregulate the ACE 2 receptor in lung (epithelial) cells – but also in vascular (endothelial) cells. This opens the door for using this micronutrient combination not just to reduce the pulmonary infection of the coronavirus but also to protect the cardiovascular system from the detrimental impact of such an infection.
The blood vessel system is the conduit of this infection to essentially all other organs of the body, which explains multi-organ failure as the cause for the high mortality rate of this pandemic. Significantly reducing the expression of viral cell entry doors on the surface of vascular endothelial cells should lead to a distinct reduction in the spread of the infection along the vascular system, minimizing the infection to other organs and, thereby, markedly reducing death rates (mortality) of infected patients.
Answering the ‘Cytokine Storm’
The advanced stage of a coronavirus infection is characterized by a mutual ‘escalation’ between the infective agent and the body’s defense system, a ‘biological battle’ that forms the basis of the inflammation. Advanced stages of coronavirus infections are characterized by excessive inflammation mediated by inflammatory cytokines. This includes an upregulation of IL-1, IL-6 and IL-10, TNF-alpha and many other cytokines, as well as an increased number of immune-competent white blood cells such as neutrophils, natural killer cells, T-helper cells and dendritic cells [Li G 2020, Chua 2020]. This intense biological communication to fight the infection has been described as a ‘cytokine storm’.
Since the number of ACE2 receptors expressed is associated with an increased inflammatory state, a decrease in the number of ACE2 proteins produced would also be associated with a decrease of inflammation. To test this connection, and to simulate the actual situation as it occurs during coronavirus infection, we stimulated lung epithelial cells with tumor necrosis factor alpha (TNF-alpha), the master regulator of the cytokine storm (Parameswaran 2010).
Our results show that in an inflammatory environment, i.e. in the presence of increased levels of TNF-alpha, micronutrients are even more effective and can lower the expression of the ACE2 receptor by more than 80% (Figure 2). Considering the fact that the ACE2 protein does not just determine the rate of viral entry into the cells but is also involved in the active secretion of inflammatory cytokines (Li G, 2020), thereby sustaining a pro-inflammatory environment, these results cannot be overestimated. The micronutrient composition tested in this study is obviously capable of interrupting the vicious cycle between coronavirus infection -> increased ACE2 receptor expression/increased viral entry -> increased production of inflammatory cytokines -> still higher expression of ACE2 receptor -> advanced inflammation and so on (Figure 4).
We are not aware of any earlier description in the scientific literature of such a distinct effect of micronutrients on the cellular ‘entry doors’ of coronavirus, especially under inflammatory conditions. These results make specific micronutrient combinations prime candidates in interrupting the frequently fatal spiral between increasing coronavirus uptake and progressive inflammation.
Answering the ‘Free Radical Storm’
There is a close connection between infection, inflammation and the production of so-called ‘oxygen free radicals’. These are highly aggressive molecules used, among others, by activated white blood cells to attack and kill viruses and other pathogens. If an infection lasts too long due to a weak immune system, a chronically elevated ‘oxidative stress’ level can cause substantial damage to body tissue and further aggravate the infectious disease. Recently, details of this biological “crosstalk” between TNF-alpha and oxygen free radicals have been elucidated (Blaser 2016).
Oxidative stress has been proposed to be an aggravating factor during coronavirus infections (Potus 2020). This observation is supported by the fact that cigarette smoking (Smith 2020) as well as air pollution (Liang 2020) – both characterized by the exposure to high levels of oxygen free radicals – have been found to be associated with an increased risk for COVID-19. Significantly, smoking has also been found to increase the expression of ACE2 receptors in human lung tissue (Cai 2020), strongly indicating that this pro-oxidative effect can be counteracted by antioxidants.
Several ingredients of the micronutrient composition tested here, including ascorbic acid, green tea polyphenols (EGCG), N-acetylcysteine and quercetin, are powerful antioxidants. These properties could be jointly responsible for the remarkable efficacy of the tested micronutrient composition reported here.
Benefit of micronutrient combinations in the fight against coronaviruses: Summary of the scientific evidence
The results of our study contribute to a better understanding of the key disease mechanisms involved in coronavirus infections. The benefits of micronutrients to counteract the progression of the disease and to qualify as essential measure to prevent future pandemics are summarized in Figure 4.
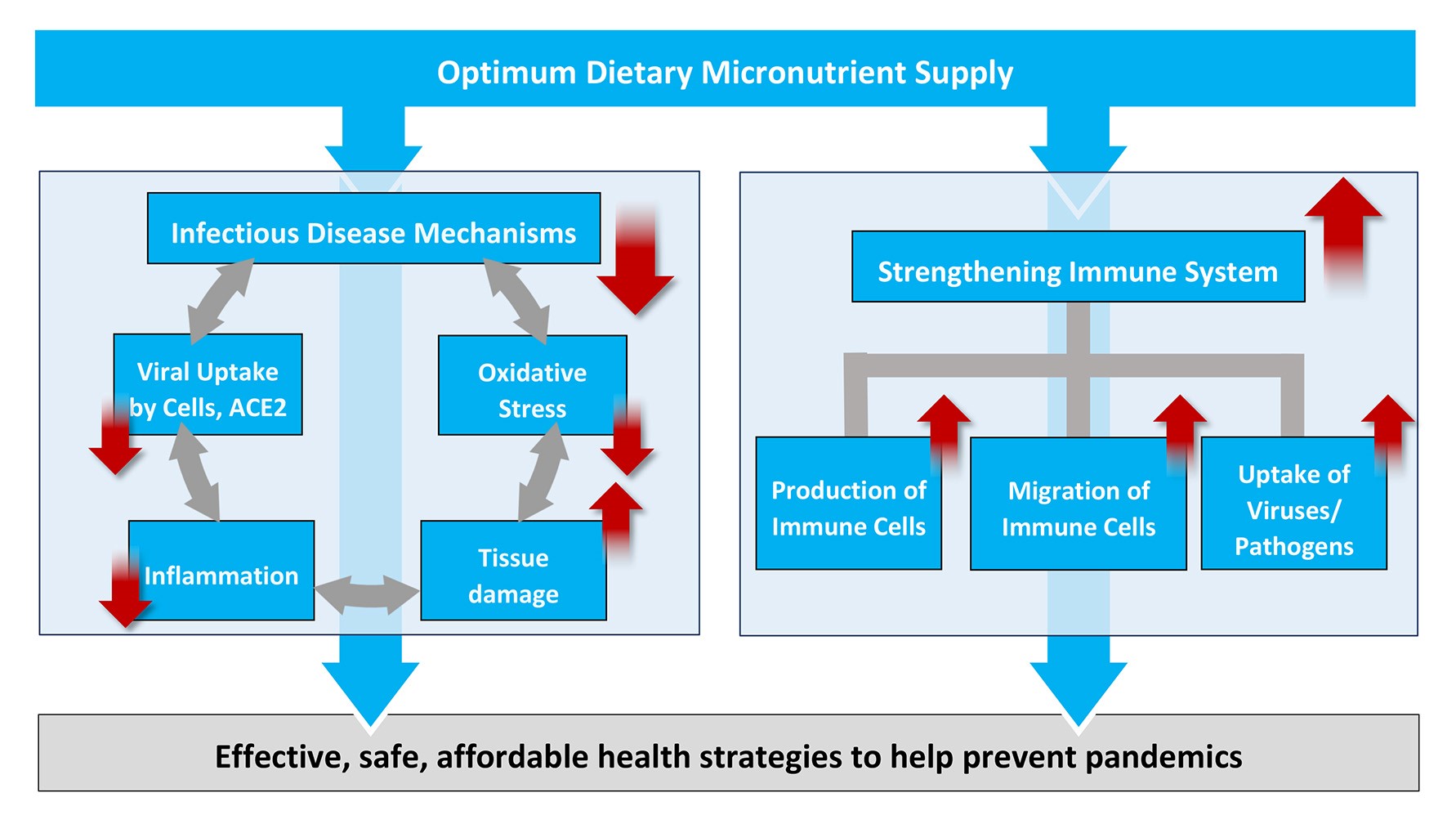 Figure 4: Scientific rationale for increased micronutrient intake as an effective, safe and affordable global health strategy to fight COVID-19 and – at the same time – to help prevent future pandemics.
Figure 4: Scientific rationale for increased micronutrient intake as an effective, safe and affordable global health strategy to fight COVID-19 and – at the same time – to help prevent future pandemics.
The connections between micronutrient deficiency and inflammation, oxidative stress and the other disease mechanisms associated with infections are documented in thousands of scientific publications accessible on pubmed.gov and elsewhere. A more detailed discussion would go beyond the scope of this publication.
A decisive benefit of the micronutrient approach presented here is the essential role of micronutrients in strengthening the immune system, from an increased production of white blood cells, to their accelerated migration towards an infection (chemotaxis) to an improved capacity to kill and remove the invaders (phagocytosis). No vaccine or synthetic drug is able to improve the overall immune response in the defense against infective agents in general.
Coronavirus pandemics as micronutrient deficiency diseases
This study identifies coronavirus pandemics as micronutrient-deficiency diseases directly or indirectly promoted by long-term suboptimal micronutrient intake. In their roles as modulators of general immune defense and their specific role in reducing the expression of cellular ‘entry doors’ for coronaviruses, these natural bioactive compounds must be considered as the basis for successful control and prevention of coronavirus pandemics.
This conclusion is further supported by the available evidence about the beneficial clinical use of vitamin C in COVID-19. Reports from China and other countries have identified high-dose intravenous vitamin C given to patients with advanced stages of COVID-19 as an effective and safe therapy (Shanghai Medical Association 2020) particularly to mitigate the ‘cytokine storm’ and improve the critical oxygenation index in patients, i.e. how much oxygen reaches the blood through the (inflamed) lung membranes. The advantage of micronutrient-based public health strategies becomes even more compelling when compared to conventional options.
New global public health strategies based on optimum micronutrient supply
Political decision takers around the world are struggling to define the right strategy in order to end the current pandemic, protect their people from future pandemics – and to limit the economic burden. Until now, the focus has mainly been on potential vaccines and so-called ‘antiviral’ drugs, considered in official recommendations by most governments as well as by the World Health organization (WHO).
Interestingly, most vaccine developments also target intercepting the docking of the virus to the ACE2 receptor by means of antibodies that attach to the binding ‘spike protein’ on the surface of the virus – or the ACE2 receptor on the surface of the human cell. If successful, this approach may possibly reduce viral entry into cells. However, these antibodies would have little or no effect on the expression of the number of ACE2 receptors since they are unable to regulate the ACE2 expression in the cell core. Since ACE2 receptors are not just the ’entry door’ for the virus, but also responsible for maintaining the disease, including intracellular virus replication (Li, G. 2020), the down-regulation of the ACE2 receptor is, logically, a superior strategy when compared to simply (sterically) blocking the already expressed receptors.
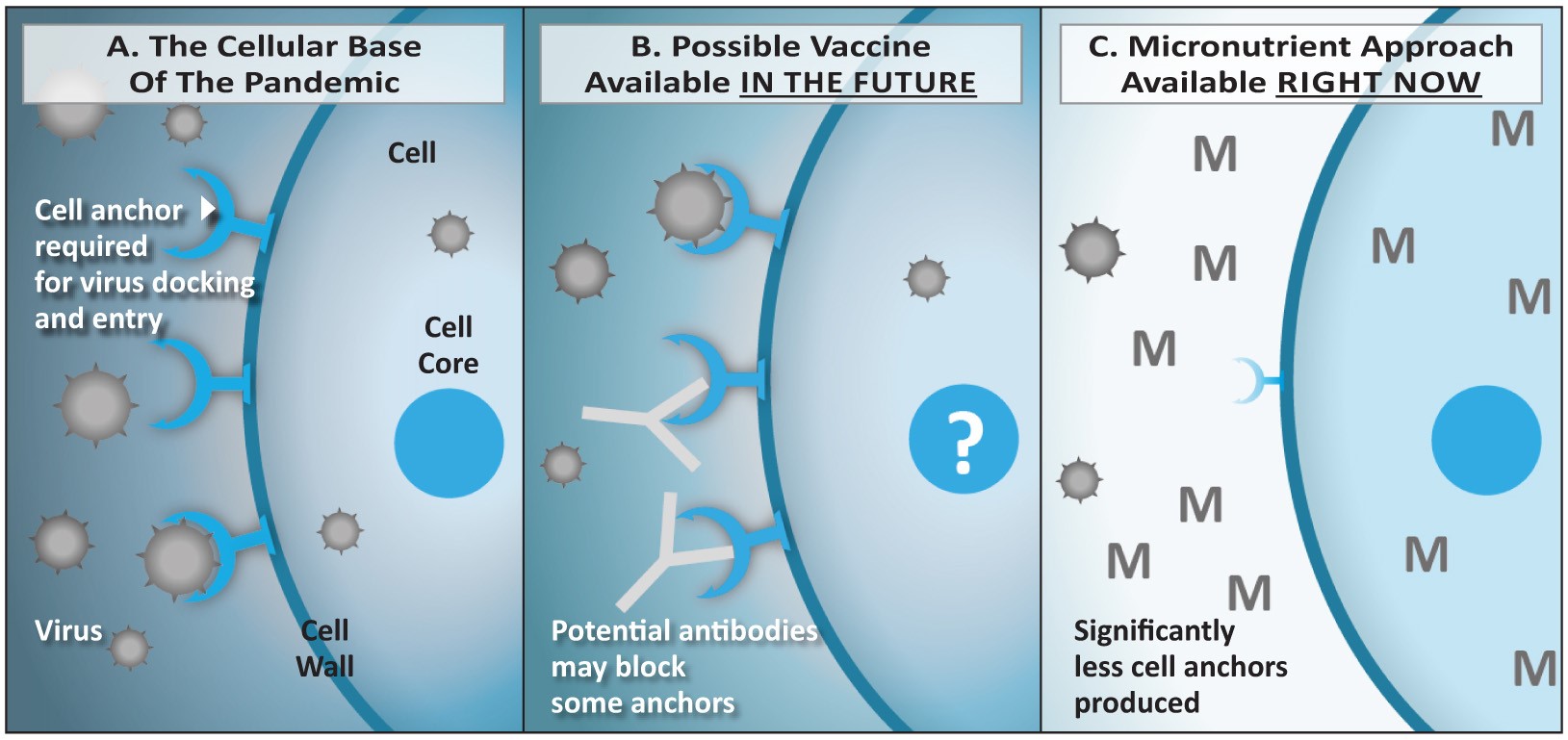 Figure 5: Comparison of strategies based on vaccines (B) versus micronutrients (C).
Figure 5: Comparison of strategies based on vaccines (B) versus micronutrients (C).
5A: The coronavirus enters the vascular endothelial and lung epithelial cells via the ACE2 receptor. The number of receptors expressed is regulated at the level of the cellular DNA in the cell core (nucleus). 5B: Vaccines induce the production of antibodies by the immune system. If successful, they could reduce the binding of the virus to the receptor and its entry into the cell. However, the vaccination would have limited effect on the number of receptors expressed by the cells. 5C: Micronutrients enter the cells and its nucleus and exert a regulatory role on the DNA that leads to a decreased expression of the ACE2 receptor on the cell surface. Thus, the ‘entry doors’ for the virus are not just blocked by an antibody, there are simply significantly less ‘entry doors’ available – or none at all.
When the available so-called antiviral drugs are included in such an assessment, the advantage of a micronutrient-based strategy becomes even more compelling. Most ‘antiviral’ drugs – including the already registered drug ‘remdesivir’– do not attack only the virus as the name would suggest, they rather interfere with the multiplication of cells in general. Thus, in taking such ‘antiviral’ drugs, the almost inevitable consequence for the patient is a decrease in the production and function of immune cells, i.e. a weakening of the immune defense. A first clinical study with such ‘antiviral’ drugs had to be terminated early, because of severe side-effects caused by the tested drug (Wang Y, 2020).
Table 2 compares the currently discussed strategies of vaccines and antiviral drugs to the micronutrient-based health strategy.
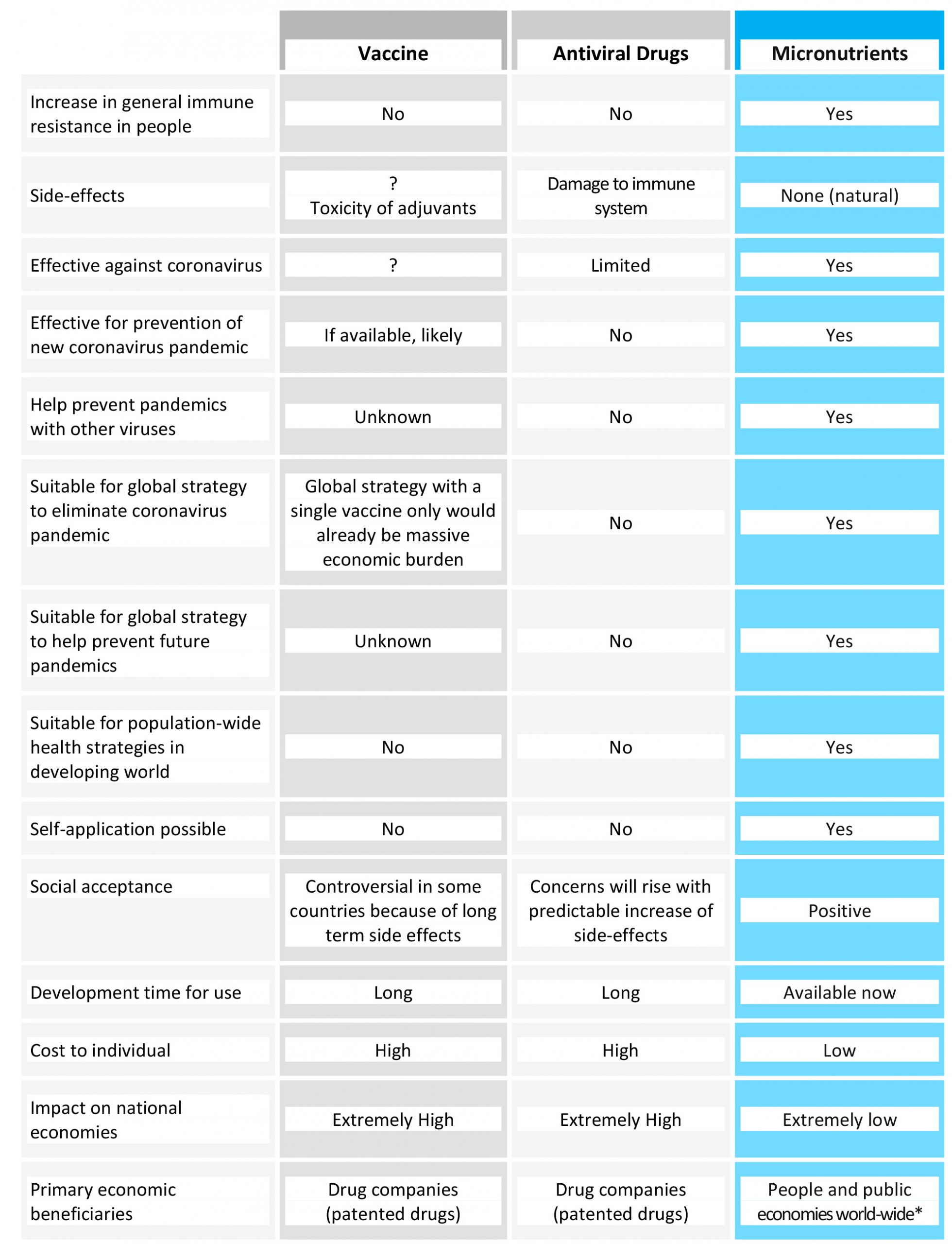 Table 2. Comparison of a long-term public health strategy based on micronutrient supply with other proposals currently discussed, namely vaccines and antiviral pharmaceutical drugs. For * see the end of the publication.
Table 2. Comparison of a long-term public health strategy based on micronutrient supply with other proposals currently discussed, namely vaccines and antiviral pharmaceutical drugs. For * see the end of the publication.
This comparison highlights the compelling reasons for micronutrient-based strategies in the fight against the current pandemic.
Potential obstacles to micronutrient-based public health strategies
The main obstacle to implementing an effective, safe and affordable micronutrient-based health strategy comes from an investment business that is built around patented synthetic drugs: the pharmaceutical industry. The lucrative license fees from patented drugs constitute the ‘return on investment’ for this industry, thereby forming the very basis of its business model.
Micronutrients are biologically active natural compounds that are generally not patentable. Their wide application, therefore, must undermine an investment business based on patented drugs. Not surprisingly, the overwhelming health benefits of vitamins and other micronutrients have been neglected in medical education and are often publicly discredited. Moreover, for almost 30 years now, an international body, the so-called ‘Codex Alimentarius Commission’, has openly pursued a worldwide ban on the therapeutic use of vitamin and mineral supplements (Taylor 2020). These decades-long efforts to sideline natural therapies may even have contributed to the rapid spread of the current pandemic: they may have prevented people around the world from accepting micronutrients as a scientifically proven way to strengthen their immunity.
In light of the human and economic costs of the current pandemic, the results of the study presented here are compelling. They may pave the way to the general acceptance of micronutrients as an integral part of modern medicine and a foundation of preventive health care. Nutrient-based cellular medicine combines wide health benefits with general safety, an argument not to be neglected in light of the fact that each year tens of thousands of people die from the side-effects of synthetic prescription drugs (Light 2020).
Unrestricted access to science-based natural health must become an inalienable human right
Mankind stands at the cross-roads. If humanity does not take advantage of the scientific facts about the overwhelming health benefits of micronutrients, the consequences will be predictable: Each new pandemic will take more human lives and will further ruin the global economy, ultimately strangulating the life of future generations both physically and economically.
Thus, absolute priority must be given to public health strategies that target the strengthening of the immune system of all people in order to boost resistance against any future infectious invader. Since micronutrients are the only scientific approach to enhance the general immune system in humans, the people around the world must spread this lifesaving health information and make sure that they continue to have free access to micronutrients. The unrestricted access to micronutrients and other science-based natural health approaches must become an inalienable human right.
Additional information
* The Dr. Rath Research Institute operates on a non-profit basis. Its research focusses on establishing the health benefits of micronutrients in the battle against cardiovascular diseases and cancer, as well as infectious disease. For the micronutrient composition described here, patents have been filed top protect the know-how. In contrast to the drug investment business, however, where patents serve as the tools for the ‘return on investment’, our patents were filed in order to protect this knowledge for mankind and prevent it from abuse for other purposes.
We are ready to license our expertise, including this micronutrient composition, to any government or publicly controlled institution anywhere in the world – free of charge. However, this knowledge cannot be directly or indirectly sublicensed to pharmaceutical drug companies, for they can have no interest in replacing their lucrative patented drugs with effective, safe and affordable micronutrients.
If you are interested in any form of collaboration, please contact our research institute at www.drrathresearch.org.
REFERENCES
Ackermann M et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. Online ahead of print (2020). PMID: 32437596, DOI: 10.1056/NEJMoa2015432
Barbour EK et al. Standardization of a new model of H9N2/escherichia coli challenge in broilers in Lebanon. Vet Ital. 2009;45(2):317-322. PMID: 20391382.
Barbour EK et al. Alleviation of histopathological effects of avian influenza virus by a specific nutrient synergy. Int J Appl Res Vet M. 2007;5(1):9-16. Research Gate Website, website of the Dr. Rath Research Institute Website.
Bavishi C et al. Acute Myocardial Injury in Patients Hospitalized With COVID-19 Infection: A Review. Prog Cardiovasc Dis. 2020;S0033-0620(20)30123-7. PMID: 32512122, PMCID: PMC7274977, DOI: 10.1016/j.pcad.2020.05.013.
Blaser H et al. TNF and ROS Crosstalk in Inflammation. Trend Cell Biol. 2016;26(4):249-261. PMID: 26791157, DOI: 10.1016/j.tcb.2015.12.002.
Cai G et al. Tobacco Smoking Increases the Lung Gene Expression of ACE2, the Receptor of SARS-CoV-2. Am J Respir Crit Care Med. 2020;201(12):1557-1559. PMID: 32329629, PMCID: PMC7301735, DOI: 10.1164/rccm.202003-0693LE.
Chua RL et al. COVID-19 Severity Correlates With Airway Epithelium-Immune Cell Interactions Identified by Single-Cell Analysis. Nat Biotechnol. Online ahead of print (2020). PMID: 32591762, DOI: 10.1038/s41587-020-0602-4.
Deryabin PG et al. Effects of a nutrient mixture on infectious properties of the highly pathogenic strain of avian influenza virus A/H5N1. Biofactors. 2008;33(2):85-97. PMID: 19346584, DOI: 10.1002/biof.5520330201.
Gurwitz D. Angiotensin Receptor Blockers as Tentative SARS-CoV-2 Therapeutics. Drug Dev Res. Online ahead of print (2020). PMID: 32129518, PMCID: PMC7228359, DOI: 10.1002/ddr.21656.
Hamming I et al. Tissue Distribution of ACE2 Protein, the Functional Receptor for SARS Coronavirus. A First Step in Understanding SARS Pathogenesis. J Pathol. 2004;203(2):631-7. PMID: 15141377, DOI: 10.1002/path.1570.
Hofmann H. et al. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc Natl Acad Sci U S A. 2005;102(22):7988-93. PMID: 15897467, DOI: 10.1073/pnas.0409465102.
Huang C et al. Clinical Features of Patients Infected With 2019 Novel Coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. PMID: 31986264. PMCID: PMC7159299, DOI: 10.1016/S0140-6736(20)30183-5.
Jariwalla RJ et al. Micronutrient Cooperation in Suppression of HIV Production in Chronically and Latently Infected Cells. Mol Med Rep. 2010;3(3):377-85. PMID: 21472250, DOI: 10.3892/mmr_00000268.
Jariwalla RJ et al. Suppression of influenza A virus nuclear antigen production and neuraminidase activity by a nutrient mixture containing ascorbic acid, green tea extract and amino acids. Biofactors. 2007;31(1):1-15. PMID: 18806304, DOI: 10.1002/biof.5520310101.
Koralnik IJ, Tyler KL. COVID-19: A Global Threat to the Nervous System. Ann Neurol. 2020;88(1):1-11. PMID: 32506549, PMCID: PMC7300753, DOI: 10.1002/ana.25807.
Lan J et al. Structure of the SARS-CoV-2 Spike Receptor-Binding Domain Bound to the ACE2 Receptor. Nature. 2020;581(7807):215-220. PMID: 32225176, DOI: 10.1038/s41586-020-2180-5.
Li G et al. Assessing ACE2 Expression Patterns in Lung Tissues in the Pathogenesis of COVID-19. J Autoimmun. Online ahead of print (2020). PMID: 32303424, PMCID: PMC7152872, DOI: 10.1016/j.jaut.2020.102463.
Li W et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450-4. PMID: 14647384, DOI:10.1038/nature02145.
Light, DW (2014) ‘New Prescription Drugs: A Major Health Risk With Few Offsetting Advantages’, EJS Center for Ethics, Harvard University, 27 June. Available at: https://ethics.harvard.edu/blog/new-prescription-drugs-major-health-risk-few-offsetting-advantages (Accessed: June 2020).
Mahmoodian F, Peterkofsky B. Vitamin C Deficiency in Guinea Pigs Differentially Affects the Expression of Type IV Collagen, Laminin, and Elastin in Blood Vessels. J Nutr. 1999;129(1):83-91. PMID: 9915880, DOI: 10.1093/jn/129.1.83.
Mosleh W et al. Endotheliitis and Endothelial Dysfunction in Patients With COVID-19: Its Role in Thrombosis and Adverse Outcomes. J Clin Med. 2020;9(6): E1862. PMID: 32549229, DOI: 10.3390/jcm9061862.
Nishikimi M et al. Cloning and Chromosomal Mapping of the Human Nonfunctional Gene for L-gulono-gamma-lactone Oxidase, the Enzyme for L-ascorbic Acid Biosynthesis Missing in Man. J Biol Chem. 1994;269(18):13685-8. PMID: 8175804.
Parameswaran N, Patial S. Tumor Necrosis factor-α Signaling in Macrophages. Crit Rev Eukaryot Gene Expr. 2010;20(2):87-103. PMID: 21133840, PMCID: PMC3066460, DOI: 10.1615/critreveukargeneexpr.v20.i2.10.
Pons S et al. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care. 2020;24(1):353. PMID: 32546188, PMCID: PMC7296907, DOI: 10.1186/s13054-020-03062-7.
Poon LLM, Peiris M. Emergence of a Novel Human Coronavirus Threatening Human Health. Nat Med. 2020;26(3):317-319. PMID: 32108160, DOI: 10.1038/s41591-020-0796-5.
Potus F et al. NOVEL INSIGHTS ON THE PULMONARY VASCULAR CONSEQUENCES OF COVID-19. Am J Physiol Lung Cell Mol Physiol. Online ahead of print (2020). PMID: 32551862, DOI: 10.1152/ajplung.00195.2020.
Shanghai Medical Association (2020) ‘Expert consensus on comprehensive treatment of coronavirus disease in Shanghai 2019’, COVID-19 World News, 4 March. Available at: https://covid19data.com/2020/03/04/expert-consensus-on-comprehensive-treatment-of-coronavirus-disease-in-shanghai-2019/ (Accessed: April 2020)
Smith JC et al. Cigarette Smoke Exposure and Inflammatory Signaling Increase the Expression of the SARS-CoV-2 Receptor ACE2 in the Respiratory Tract. Dev Cell. 2020;53(5):514-529.e3. PMID: 32425701, PMCID: PMC7229915, DOI: 10.1016/j.devcel.2020.05.012.
Tai W et al. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. Online ahead of print (2020). PMID: 32203189, DOI: 10.1038/s41423-020-0400-4.
Taylor, PA (2020) 'The Codex Alimentarius Commission: The Facts That Everyone Needs To Know', Dr. Rath Health Foundation, 5 June. Available at: https://www.dr-rath-
Yan R et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444-1448. PMID: 32132184, DOI: 10.1126/science.abb2762.
Vlahos R et al. Inhibition of Nox2 Oxidase Activity Ameliorates Influenza A Virus-Induced Lung Inflammation. PLoS Pathog. 2011;7(2):e1001271. PMID: 21304882, PMCID: PMC3033375, DOI: 10.1371/journal.ppat.1001271.
Varga Z et al. Endothelial cell involvement and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417-1418. PMID: 32325026, DOI: 10.1016/S0140-6736(20)30937-5.
Wan Y et al. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol. Online ahead of print (2020). PMID: 31996437, DOI: 10.1128/JVI.00127-20.
Wang C et al. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470-473. PMID: 31986257, DOI: 10.1016/S0140-6736(20)30185-9.
Wang LF et al. From Hendra to Wuhan: what has been learned in responding to emerging zoonotic viruses. Lancet. 2020;395(10224): e33–e34. PMID: 32059799, PMCID: PMC7133556, DOI: 10.1016/S0140-6736(20)30350-0.
Wang Y et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569-1578. PMID: 32423584, PMCID: PMC7190303 DOI: 10.1016/S0140-6736(20)31022-9.
Wit E et al. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523-34. PMID: 27344959, DOI: 10.1038/nrmicro.2016.81.
Zhou P et al. A Pneumonia Outbreak Associated With a New Coronavirus of Probable Bat Origin. Nature. 2020;579(7798):270-273. PMID: 32015507, PMCID: PMC7095418, DOI: 10.1038/s41586-020-2012-7.
Zhu N et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733. PMID: 31978945, DOI: 10.1056/NEJMoa2001017.